What are acids and bases?
Acids and bases
What are acids and bases?
In chemistry two different types of substances are called acids and bases that are opposed to each other. Each of these substances has specific properties that modify the behavior of chemical solutions. Both acids and bases can be found in liquid, gaseous and solid states (the powder).
When acids and bases come together in a solution, an exothermic reaction occurs, that is, heat is produced. This reaction is known as neutralization.
What is an acid?
Acids are those substances that release positive hydrogen ions (H +) in a solution. This definition was introduced by scientist Svante Arrhenius.
Another concept, developed by scientist Gilbert Newton Lewis, defines acids as substances that can receive or absorb a pair of electrons from solution.
As examples of acids we can mention the following:
- Acetic acid or CH 3 COOH (vinegar);
- ascorbic acid or C 6 H 8 O 6 (vitamin C);
- phosphoric acid or H 3 PO 4 (present in soft drinks);
- lactic acid or C 3 H 6 O 3 (produced during physical exercise);
- citric acid or C 6 H 8 O 7 (oranges, grapefruits, lemons, tangerines, etc.).
Characteristics of acids
Among the characteristics or properties of acids we can mention the following:
- They have the ability to destroy organic tissues.
- They produce reactions by interacting with certain metals.
- They act as conductors of electrical current.
- When mixed with bases they produce water and salt.
- They are sour to taste.
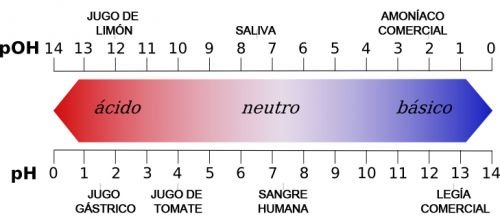
- The pH of acids ranges from 0 to 7 (where 7 is neutral).
- They are usually soluble in water.
Types of acids
- Strong acid: it is the one that gives up most of its hydrogen ions in solution, which means that it ionizes very easily. For example, HCl or hydrochloric acid.
- Weak acid: unlike the previous one, weak acid in aqueous solution releases H + ions to a lesser extent. For example, acetic acid.
What is a base?
According to Svante Arrhenius, bases are those substances that can capture hydrogen ions in solution or release negative ions, called hydroxyl (OH-).
Bases are also defined as those substances that contribute two electrons to the solution, following the theory of Gilbert Newton Lewis.
As an example of bases , we can mention the following:
- Sodium hydroxide or NaOH (caustic soda);
- potassium hydroxide or KOH (soap);
- aluminum hydroxide or Al (OH) 3 (stomach antacid);
- magnesium hydroxide or Mg (OH) 2 (milk of magnesia);
- calcium hydroxide or CaOH (lime).
Characteristics of the bases
Among the characteristics or properties of the bases we can mention:
- They slide to the touch when they are in solution, that is, they are soapy (like bleach).
- They do not react to contact with metals.
- They are conductors of electrical current in solution.
- When mixed with acids they produce water and salt.
- They are bitter to taste.
- The pH of the bases ranges from 7 to 14 (where 7 is neutral).
- Some bases are insoluble.
Types of bases
In the field of bases, at least two elementary types are known:
- Strong base: Refers to a variety of electrolyte that is attributed a strong character and can therefore be fully ionized in an aqueous solution. For example, caustic soda.
- Weak base : refers to those bases that do not fully dissociate in aqueous solution, resulting in the presence of an OH ion plus the basic radical. For example, ammonia or ammonium hydroxide.
Difference between acids and bases
One of the most important differences between acids and bases is that acids take up electrons from the solution in which they are dissolved, while bases provide them. Also, acids release positive hydrogen ions, while bases release hydroxyl.
Due to these differences, acids and bases produce different effects in chemical solutions. For example, it is customary to use litmus paper in pH tests. The blue iridescent paper acquires warm tones when in contact with acids, that is, it acquires pink or red tones depending on the intensity. On the contrary, when a base reacts with a reddish iridescent paper, it acquires blue tones.
- Update date: March 6, 2021.