What is atmospheric pressure?
Atmospheric pressure
What is atmospheric pressure?
Atmospheric pressure or barometric pressure is the force exerted by the air column of the atmosphere on the earth's surface at a certain point.
This force is inversely proportional to altitude. The higher the altitude, the lower the atmospheric pressure, and the lower the altitude, the higher the atmospheric pressure.
The highest atmospheric pressure is that which occurs at sea level. Therefore, this measurement is taken as a reference of normal atmospheric pressure .
Atmospheric pressure units
There are several units of measurement to represent atmospheric pressure. The one used in the SI is called Pascal (Pa) or hectopascal (hPa). However, bars (b), millibars (mb), “atmospheres” (atm), millimeters of mercury (mm Hg) and Torricellis (Torr) are also used.
Atmospheric pressure formula
The formula for calculating atmospheric or barometric pressure is governed by the principles of the fundamental hydrostatic equation . Let's see next.
Pa = ρ.gh
In this formula,
- Pa is equal to the pressure exerted at a point in the fluid.
- ρ is equal to the density of the fluid.
- g is equal to the acceleration of gravity.
- h equals depth.
Thus, if:
- ρ = 13550 kg / m3 (density of mercury)
- g = 9.81 m / s2
- h = 0.76 m (height of the mercury column)
Then,
- Pa = 101 023 Pa
Value of atmospheric pressure at sea level
The normal atmospheric pressure value (at sea level) is 760 mm, which is equivalent to 760 torr; at 1,013.2 mb (millibars); 101 325 Pa (pascals); at 1013.2 hPa (hectopascals) or also at 1 atm (atmosphere).
Instrument for measuring atmospheric pressure
 Arenoid barometer
Arenoid barometer
The instrument for measuring atmospheric pressure is known as a barometer . That is why atmospheric pressure is also called barometric pressure. There are several types of barometers. The most important are the following:
Mercury barometer
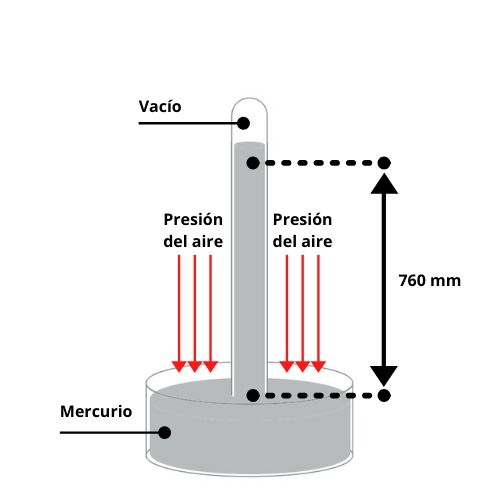 Basic scheme of the mercury barometer.
Basic scheme of the mercury barometer.
It is the first barometer in history, invented by Evangelista Torricelli in 1643. It is a glass tube open at the lower end and closed at the upper end. This tube is filled with mercury, the level of which varies according to the weight of the column of air that rests on the device. Let's see how it is possible.
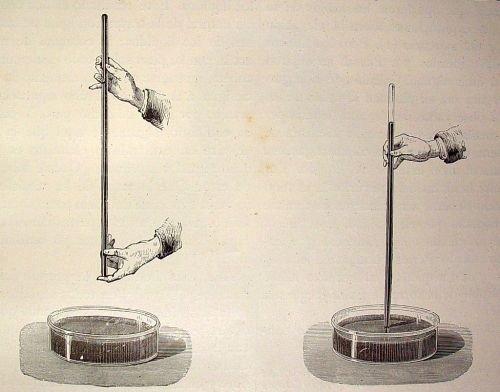
In the so-called Torricelli experiment, the scientist completely filled a one-meter-long tube with mercury and closed it with a finger. Then he turned it over, placed it at a certain inclination in a container, also with mercury, and released the mouth of the tube.
In doing this, the liquid descended, but the descent stopped at a height of 76 cm, creating a vacuum at the upper end. From this it was induced that the pressure in a vacuum is equal to 0. With these data, Torricelli was able to calculate the atmospheric pressure.
Arenoid barometer
 Internal mechanism of an arenoid barometer
Internal mechanism of an arenoid barometer
Invented in 1843 by Lucien Vidie, this barometer consists of a silver metallic capsule. This capsule is in contact with a lever attached to gears which, in turn, are attached to an indicator needle. The capsule contracts when there is higher pressure or expands when there is less pressure, which drives the movement of the gears and activates the indicator needle.
- Update date: March 6, 2021.
Recommended Contents